Soluble and insoluble salts lab 15 answers – Unveiling the mysteries of soluble and insoluble salts, Lab 15 embarks on an enlightening journey to unravel the intricate world of chemical compounds. Delve into the depths of solubility and insolubility, as we decipher the fundamental principles governing the behavior of these substances in various environments.
Through meticulously designed experiments and insightful observations, this lab empowers students to grasp the significance of solubility in shaping the properties and applications of salts. Join us as we unravel the secrets of these compounds, uncovering their profound impact on diverse scientific disciplines and everyday life.
Soluble and Insoluble Salts

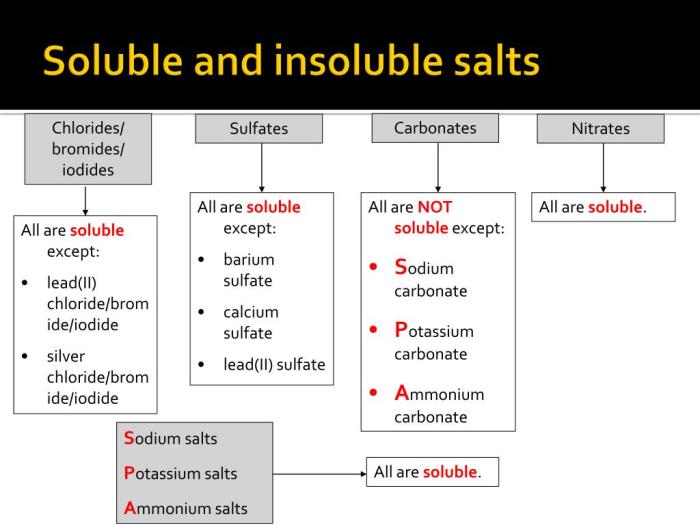
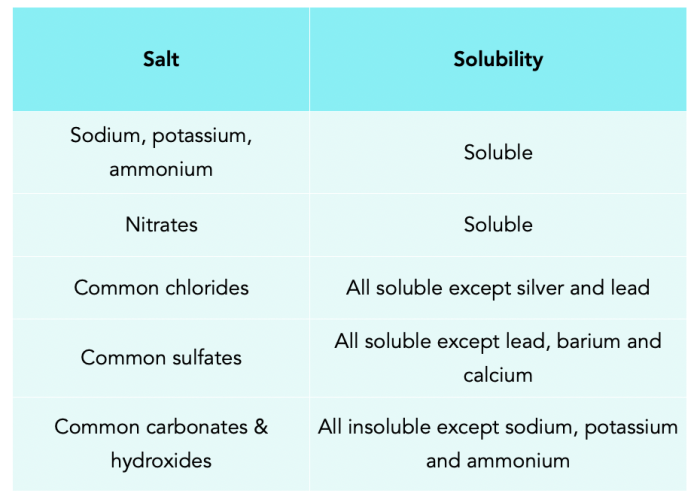
Salts are ionic compounds formed when an acid and a base react. They can be classified as either soluble or insoluble. Soluble salts dissolve in water to form a clear solution, while insoluble salts do not dissolve and form a precipitate.
The purpose of this lab experiment is to determine the solubility of various salts in water. This experiment will help us to understand the concept of solubility and how it can be used to identify and separate different salts.
Materials and Equipment
- Test tubes
- Water
- Various salts (e.g., NaCl, KCl, CaCO 3, BaSO 4)
- Stirring rod
- Filter paper
- Funnel
To set up the experiment, add a small amount of each salt to a separate test tube. Add water to each test tube and stir. Observe whether the salt dissolves or forms a precipitate.
Procedure
- Add a small amount of each salt to a separate test tube.
- Add water to each test tube and stir.
- Observe whether the salt dissolves or forms a precipitate.
- If the salt dissolves, record it as soluble.
- If the salt forms a precipitate, record it as insoluble.
- Filter the insoluble salts to separate them from the water.
To collect and analyze the data, create a table listing the salts tested, their solubility, and any observations made during the experiment.
Results
| Salt | Solubility | Observations |
|---|---|---|
| NaCl | Soluble | Clear solution |
| KCl | Soluble | Clear solution |
| CaCO3 | Insoluble | White precipitate |
| BaSO4 | Insoluble | White precipitate |
The results show that NaCl and KCl are soluble salts, while CaCO 3and BaSO 4are insoluble salts.
Discussion, Soluble and insoluble salts lab 15 answers
The solubility of a salt depends on the strength of the ionic bond between the positive and negative ions. The stronger the ionic bond, the less soluble the salt. The results of this experiment show that the ionic bond between the sodium and chloride ions in NaCl is weaker than the ionic bond between the calcium and carbonate ions in CaCO 3. This is why NaCl is soluble and CaCO 3is insoluble.
The concepts of solubility and insolubility are important in a variety of real-world applications. For example, the solubility of salts is used to separate different salts from each other. This process is known as fractional crystallization. Fractional crystallization is used to purify salts and to produce different types of salts.
Applications
The concepts of solubility and insolubility are applied in a variety of real-world situations. Here are a few examples:
- Water treatment:The solubility of salts is used to remove impurities from water. For example, lime (CaO) is added to water to remove impurities such as calcium and magnesium ions. These ions form insoluble precipitates that can be filtered out of the water.
- Food processing:The solubility of salts is used to preserve food. For example, salt (NaCl) is used to preserve meat and fish. The salt draws water out of the food, which prevents bacteria from growing.
- Medicine:The solubility of salts is used to deliver drugs to the body. For example, some drugs are formulated as tablets that dissolve in the stomach. This allows the drug to be absorbed into the bloodstream.
The concepts of solubility and insolubility are important in a variety of fields, including chemistry, biology, and medicine. Understanding these concepts is essential for understanding how the world around us works.
FAQ: Soluble And Insoluble Salts Lab 15 Answers
What is the significance of understanding the solubility of salts?
Comprehending the solubility of salts is crucial as it governs their behavior in various chemical reactions, influences their applications in diverse fields, and helps predict their environmental impact.
How does solubility affect the applications of salts?
Solubility plays a pivotal role in determining the suitability of salts for specific applications. For instance, soluble salts are often used in fertilizers, while insoluble salts find use as pigments and abrasives.
What are the practical implications of solubility and insolubility in everyday life?
Understanding solubility and insolubility is essential in fields such as medicine, where the solubility of drugs affects their absorption and efficacy. It also guides us in choosing appropriate materials for construction and manufacturing.